Betadex sulfobutyl ether sodium manufacturing is our area of expertise. It is a top pharmaceutical manufacturer.
Chinese ingredients.
Name: sodium betadex sulfobutyl ether
DMF No. 030167/USP Standard/Injection grade
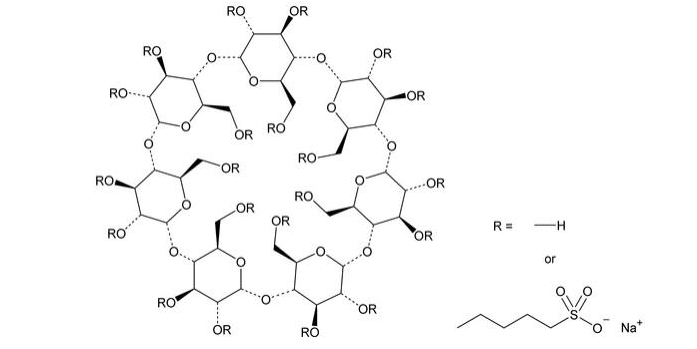
Name: Injection grade betadex sulfobutyl ether sodium
CAS: 182410-00-0
Abbreviation: SBEBCD
Molecular formula : C42H70-nO35 (C4H8O3S Na)n
Molecular weight: 1135+158n
Standard : USP-NF 2021
Package: 1kg/bag , 2kg/bag, 10kg/bag, 20kg/bag
Use: Medical
| Betadex Sulfobutyl Ether Sodium | |
| Test Items | USP Standard |
| Clarity of Solution | A 30%w/v solution in water is clear |
| Identification (IR) | Same absorption bands as USP betadex sulfobutyl ether sodium RS |
| Identification(Sodium) | Identify test are positive for Sodium |
| Identification(LC) | the retention time of the major peak of sample solution corresponds to the standard solution |
| Average Degree of Substitution | 6.2-6.9 |
| Water solution pH | pH of 30%w/v solution in water is 4.0-6.8 |
| Water content | ≤10.0% |
| β-Cyclodextrin Content | ≤0.1% |
| Sodium Chloride | ≤0.2% |
| 1,4-butane sultone | ≤0.5ppm |
| 4-Hydroxybutane-1-Sulfonic Acid | ≤0.09% |
| Disodium bis-(4-sulfobtyl) ether | ≤0.05% |
| Assay | 95.0%-105.0% |
| Bacterial endotoxins | ≤20EU/g |
| Microbiology | |
| TAMC ( cfu/g) | ≤100 cfu/g |
| TYMC ( cfu/g) | ≤50 cfu/g |
| Escherichia Coli | Absence |
1. It is a crucial modified form of beta-cyclodextrin synthesis.
2. It serves as an excipient mostly in azotic medications.
3. It is a brand-new class of highly soluble, anionic cyclodextrin derivatives.
4.It is effective at incorporating drug molecules to create covalent compounds, which enhance medication stability, water solubility, security, minimize kidney toxicity, moderate drug hemolysis, control drug release rate, and mask bed odor.
5. It has been used for oral, nasal, injectable, and topical medications.
Drugs have a specific affinity and inclusion for nitrogen.







